The collection, processing and use of medical research data generally requires the informed consent of the data subject for a specific purpose, known as informed consent (IC) (see Art. 6-11 GDPR). In addition to this classic opt-in approach (with IC), legal requirements (e.g. GDNG, KFRG, ePA) may permit the collection and use of data without the prior consent of data subjects (opt-out). In both cases, however, the obligation to provide evidence to supervisory authorities (Art. 5 para. 2 GDPR) and the data subject’s will not to participate (withdrawal, objection) should be taken into account. Therefore, in the context of constantly growing national and international research initiatives, a reliable and efficient procedure for the digital management of the data subject’s will is indispensable as the basis for legally permissible data processing.
The collection, processing and use of medical research data generally requires the informed consent of the data subject for a specific purpose, known as informed consent (IC) (see Art. 6-11 GDPR). In addition to this classic opt-in approach (with IC), legal requirements (e.g. GDNG, KFRG, ePA) may permit the collection and use of data without the prior consent of data subjects (opt-out). In both cases, however, the obligation to provide evidence to supervisory authorities (Art. 5 para. 2 GDPR) and the data subject’s will not to participate (withdrawal, objection) should be taken into account. Therefore, in the context of constantly growing national and international research initiatives, a reliable and efficient procedure for the digital management of the data subject’s will is indispensable as the basis for legally permissible data processing.
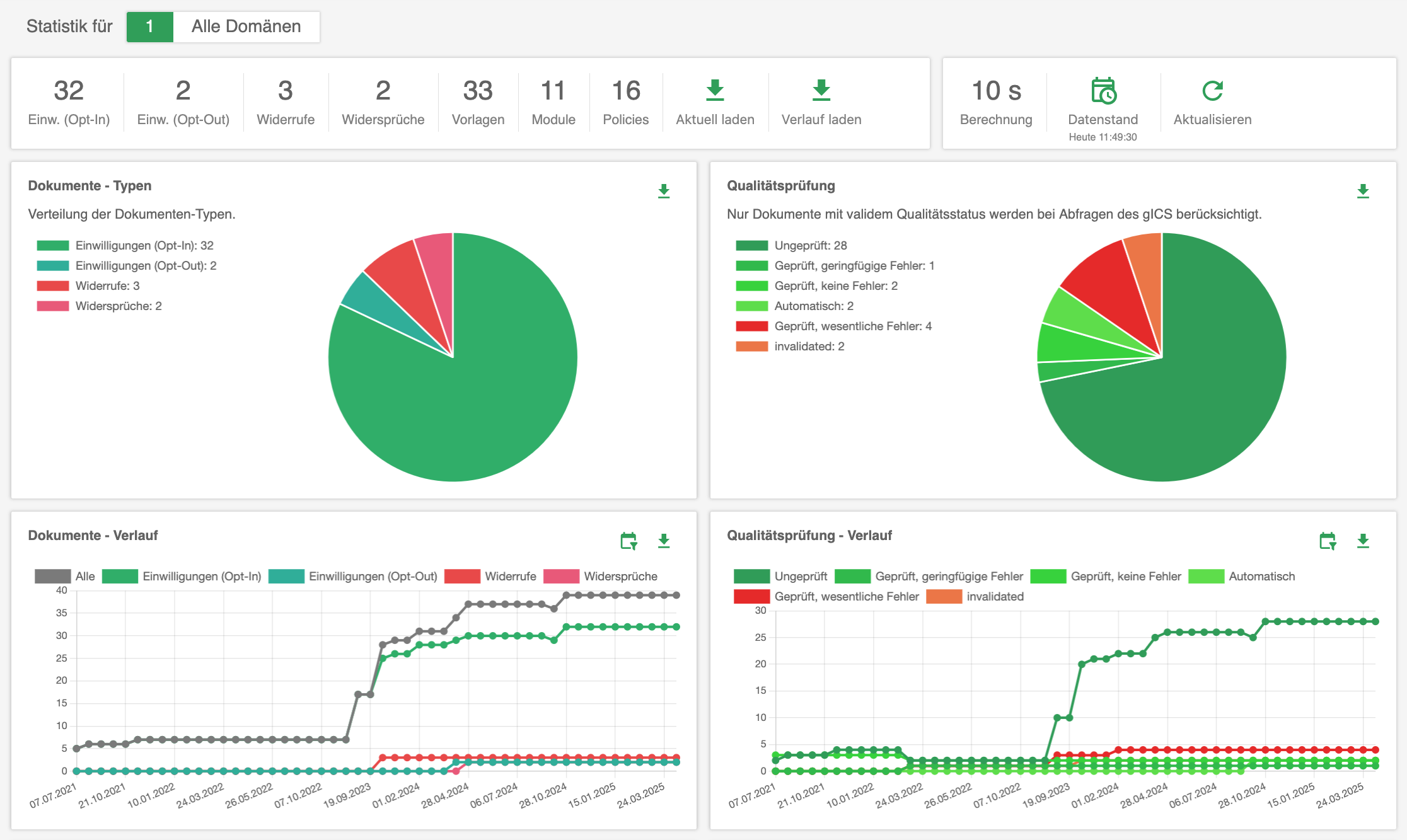
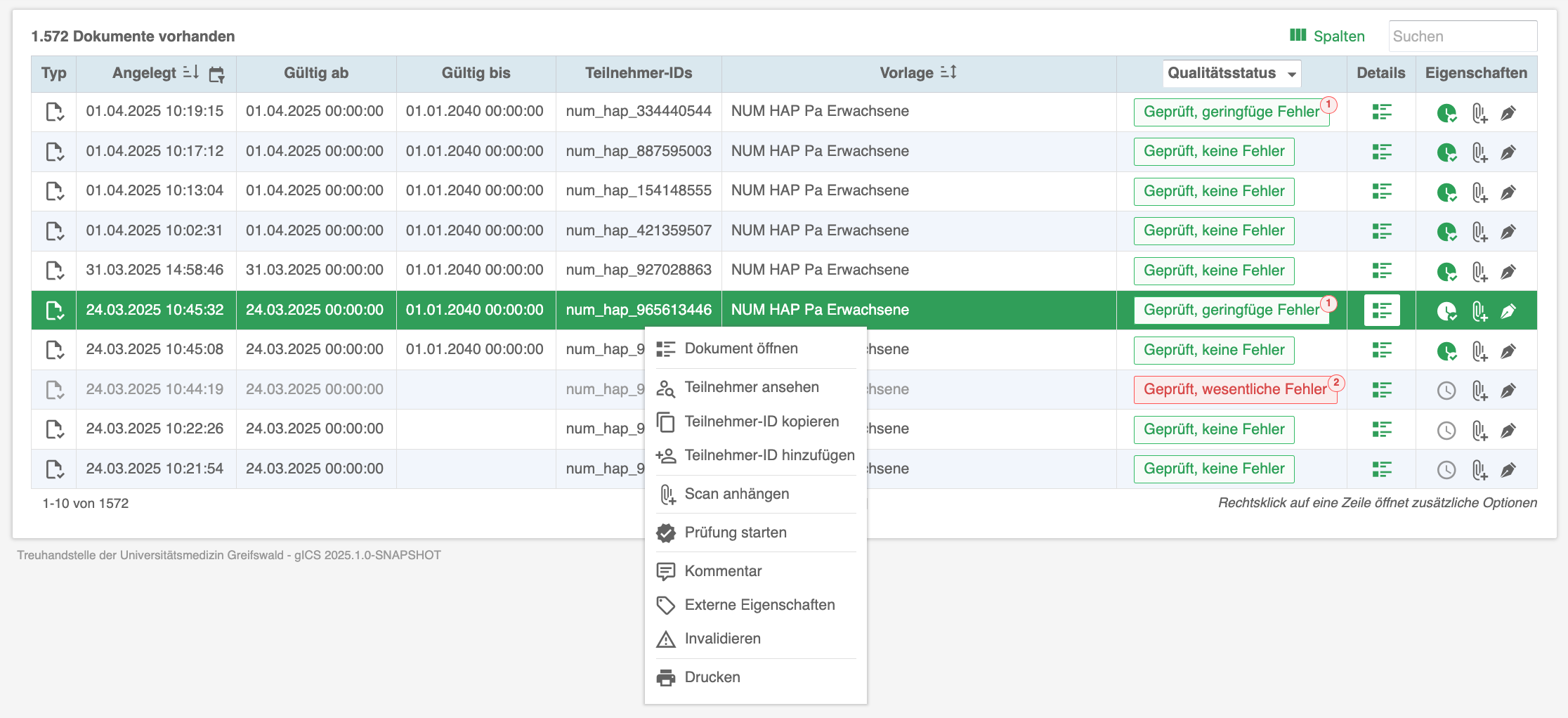
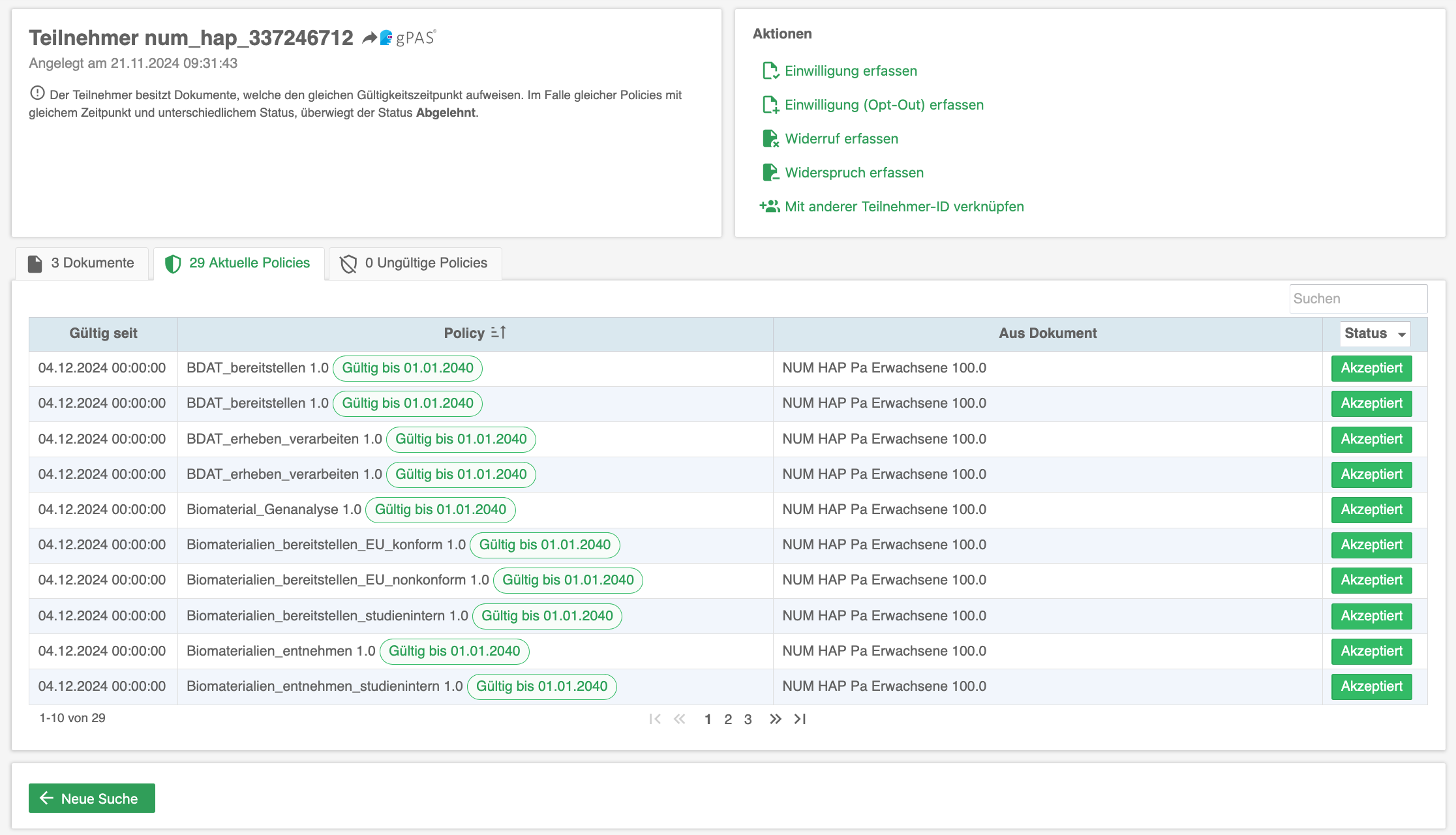
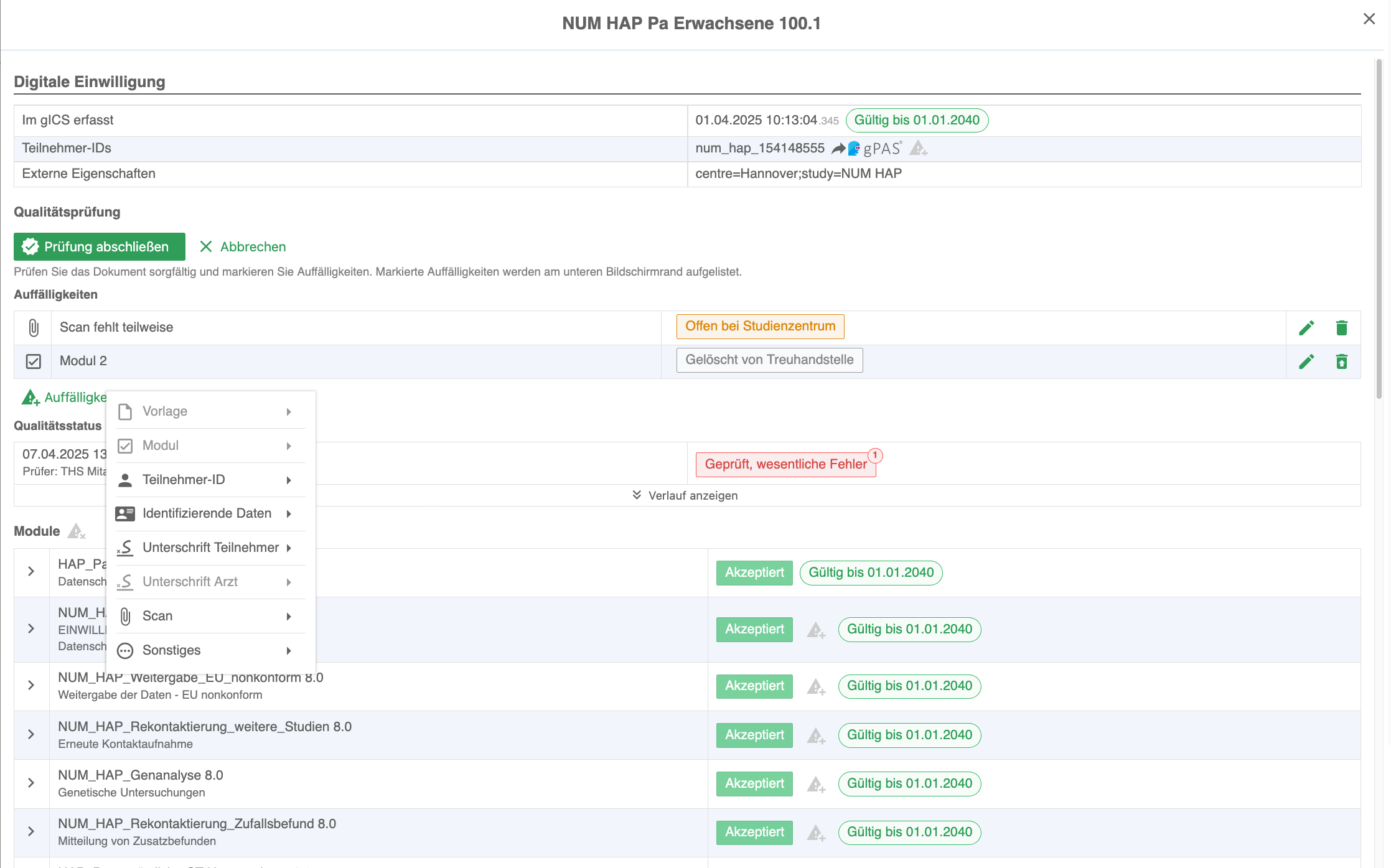
The consent management solution gICS® supports both approaches: opt-in and opt-out. gICS® can be integrated into purely paper-based consent processes (import of scans and structured documentation) and supports the necessary quality assurance. Purely digital workflows (digital signature, tablet integration, API) can also be realised quickly and easily. Comprehensive analysis mechanisms allow all consent-related documents of a data subject to be analysed in real time in a traceable and reproducible manner at any time and the valid consent status to be determined.
Templates for required documents (consents, withdrawals, objections, etc.) can be created, edited and exported or imported in the WYSIWYG editor. A wide range of response options, freely configurable additional fields, individual validity specifications (from/to date, period, age) and the option of storing properties of external systems allow a wide range of requirements for research projects and registry projects to be implemented.
Download
Are you interested in the gICS? You can download it here and simply start it using Docker. Alternatively, you can try out the gICS in our live demo.
Interfaces
Documentation
User projects
The solutions of the Trusted Third Party of the University Medicine Greifswald are becoming increasingly widespread in the community. More and more consortia, sites and projects have made a conscious decision to use our solutions to realise their individual application scenarios. Users include registries and cancer registries, local trust centres of the MII and the NUM, various study projects and selected DZGs.
We have created the THS Community Dialogue to exchange experiences and share results with each other.
Funding and publications
The gICS® is being developed by the University Medicine Greifswald (Institute of Community Medicine) and was published in 2014 as part of the MOSAIC project (funded by the DFG HO 1937/2-1). Selected functions of gICS® were realised in MAGIC (funded by the DFG HO 1937/5-1), as well as within the context of MIRACUM (funded by the BMBF 01ZZ1801M) and NUM (funded by the BMBF 01KX2021). The gICS® is continuously being enhanced on the basis of project-specific requirements and feedback from the user community.
Selected publications
- MOSAIC – A Modular Approach to Data Management in Epidemiological Studies
- MAGIC: once upon a time in consent management—a FHIR® tale
- #consented – A semantic consent code to facilitate consistent documentation and implementation of consent in collaborative medical research
- A FHIR has been lit on gICS: facilitating the standardised exchange of informed consent in a large network of university medicine