If you are already taking part or have recently received an invitation to take part in a study and are now wondering how your data are processed, you can find detailed information on the tasks of the Trusted Third Party here.
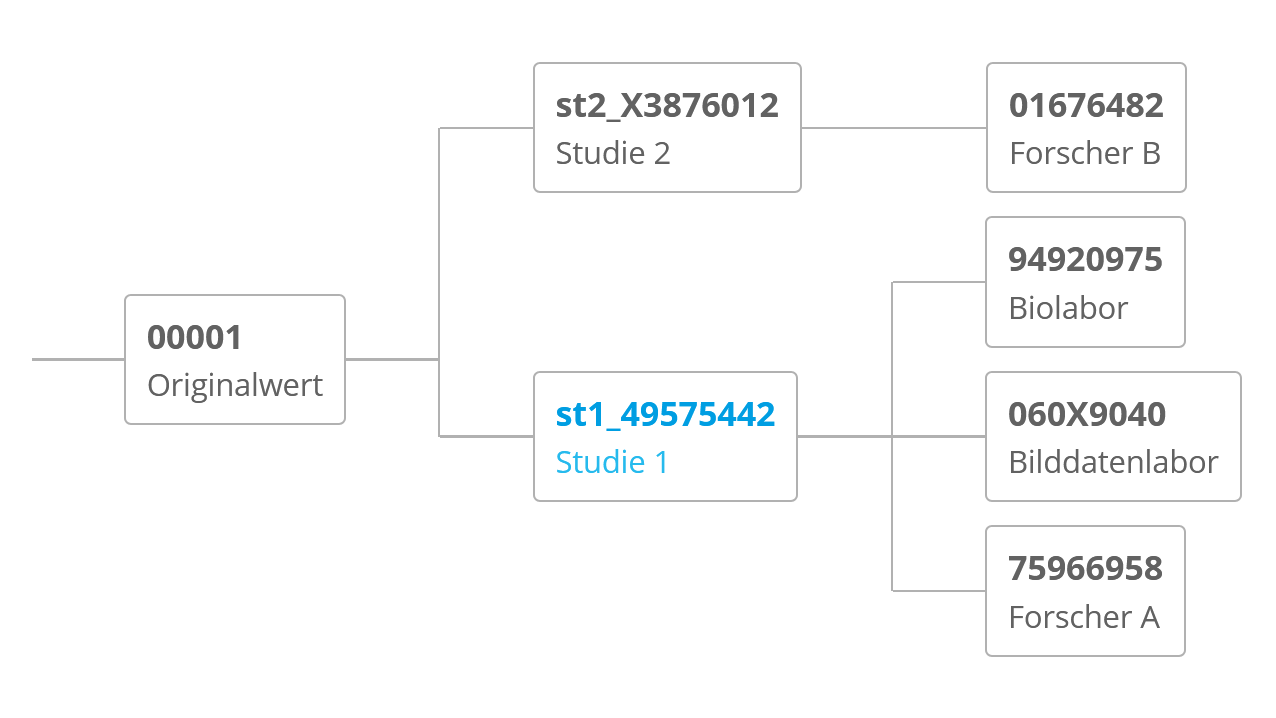
The Trusted Third Party only processes person-identifying data, such as names and addresses, based on comprehensive data protection regulations. Your data is assigned to a set of pseudonyms, which are related to you individually and exclusively. All medical data is processed using those pseudonyms only, so that identification is only possible in the Trusted Third Party. In addition, in many projects the modular consents are recorded electronically and, if necessary, archived with a scan of your paper-based consent.
This data management contributes to a clear identification of your person, as well as to the exclusion of duplicate data records. Furthermore, your pseudonymised data can be transfered to interested research groups on the basis of your daily consent status.
With your participation you make an outstanding contribution to medical research and help to improve patient care in the long term.
Do you have questions about the handling of your data in a specific project?